Prevention of Tumor Growth and Dissemination by In Situ Vaccination with Mitochondria-Targeted Atovaquone
Huang Mofei, Xiong Donghai, Pan Jing, Zhang Qi, Wang Yian, Myers Charles R., Johnson Bryon D., Hardy Micael, Kalyanaraman Balaraman, You Ming
Advanced Science, 9 2101267 (2022)
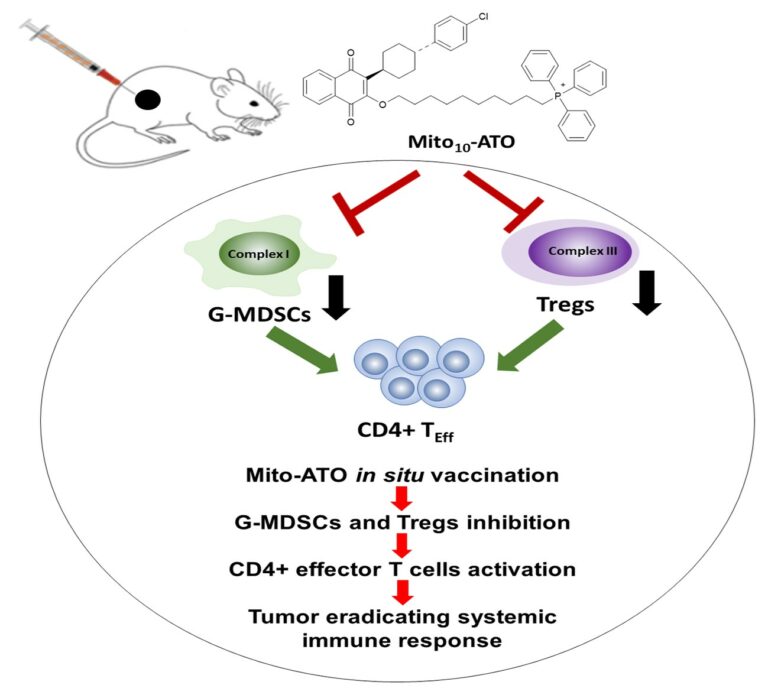
Abstract Atovaquone, an FDA-approved drug for malaria, is known to inhibit mitochondrial electron transport. A recently synthesized mitochondria-targeted atovaquone increased mitochondrial accumulation and antitumor activity in vitro. Using an in situ vaccination approach, local injection of mitochondria-targeted atovaquone into primary tumors triggered potent T cell immune responses locally and in distant tumor sites. Mitochondria-targeted atovaquone treatment led to significant reductions of both granulocytic myeloid-derived suppressor cells and regulatory T cells in the tumor microenvironment. Mitochondria-targeted atovaquone treatment blocks the expression of genes involved in oxidative phosphorylation and glycolysis in granulocytic-myeloid-derived suppressor cells and regulatory T cells, which may lead to death of granulocytic-myeloid-derived suppressor cells and regulatory T cells. Mitochondria-targeted atovaquone inhibits expression of genes for mitochondrial complex components, oxidative phosphorylation, and glycolysis in both granulocytic-myeloid-derived suppressor cells and regulatory T cells. The resulting decreases in intratumoral granulocytic-myeloid-derived suppressor cells and regulatory T cells could facilitate the observed increase in tumor-infiltrating CD4+ T cells. Mitochondria-targeted atovaquone also improves the anti-tumor activity of PD-1 blockade immunotherapy. The results implicate granulocytic-myeloid-derived suppressor cells and regulatory T cells as novel targets of mitochondria-targeted atovaquone that facilitate its antitumor efficacy.